Balancing Net Ionic Redox Equations
View Balancing Net Equations for Redox Reactionspdf from CHEMISTRY 031 at Malayan Colleges Laguna. The balanced equation will be calculated along with the states complete ionic equation net ionic equation and spectator ions.
Oxidation Reduction Reactions Redox
Replace immutable groups in compounds to avoid ambiguity.

. We will use two equations as examples. It winds up with the equation balanced in basic solution. First bromine reacts with OH ions to form bromide ions and BrO ions.
What are the balanced net-ionic equations for both of these reactions. There are several methods. Cr 2 O 7 2 Cl --- Cr 3 Cl 2 O 2 there is a minimum of 2 Crs 2Cr 6 6e --- 2Cr 3.
Check that both sides of the equation have the same kind and number of atoms as well as the same charges. In this reaction you show the nitric acid in the ionic form because its a strong acid. Similarly it also balances the number of charges ions and atoms at both sides of the equation to help you understand the reaction more easily.
Full reaction balanced in acid. The above equation takes place in two stages. There is an additional part to balancing redox reactions in acidic or basic conditions which results from the presence of oxygen and hydrogen atoms.
The following unbalanced net ionic equation provides an example. Convert the unbalanced redox reaction to the ionic form. Otherwise you may not be successful in balancing redox equations.
Use the rules to assign oxidation numbers to all atoms in the equation. Balance the following equations of redox reactions. How could you use a precipitation reaction to separate each of the following pairs of anionsWrite the formula for each reactant you would add and write a balanced net ionic equation for each reactiona Cl- and NO3-b S2- and SO42-c SO42- and CO32-d OH- and ClO4-.
Add the two half-reactions together and cancel out common terms. Balance the Redox Reaction in Acidic Medium by Half. Finally for the specified chemical equation a window will pop with the output.
1 First reaction to balance. Br 2 OH --- Br BrO. In the example above there are only two elements which simplifies balancing the reaction.
A Assign oxidation numbers for each atom. Counting of oxidation number. C Combine these redox couples into two half-reactions.
Identification and balancing of two half reactions. ELECTROCHEMISTRY Electrochemistry BALANCING NET IONIC EQUATIONS FOR REDOX REACTIONS Writing ionic. To convert it into molecular form 8 NO 3 ions are added on both side of equation.
5 A more detailed discussion about balancing this equation can be found here. Fe Au Co Br C O N F. B Identify and write out all redox couples in reaction.
Separate the process into half reactions. Guidelines for balancing redox equations. The example below shows you how to use the ion-electron method to balance this redox equation.
The balancing redox reactions calculator tells whether a reaction is actually a redox reaction or not. Solution for Balance the net ionic redox equations by the half-reaction method. Identify which atoms are oxidized and which are reduced.
This is balanced ionic equation. Balancing Redox Equations for Reactions in Basic Conditions Using the Half-reaction Method. Balancing a redox equation in acidic solution.
The unbalanced reaction isNaBr aq H2SO4 yields Br2 l SO2 g H2O l Na2SO4 aqBalance the redox reaction and write a balanced net ionic equation. Because the charge and the atoms are balanced the equation is correctly balanced. Introduction to acidbase reactions.
6 I once saw an unusual method to balancing this particular example equation. Use uppercase for the first character in the element and lowercase for the second character. For the reaction between NO 2 and H 2 the net charge on both sides of the equation in Step 1 is zero.
Balancing charge by adding electrons. Balancing Net Ionic Equations for Redox Reactions. Show all work balanced half reactions oxidation number etc used to balance the molecularequation and all work used to obtain the net ionic equationPLEASE HELP.
This is balanced oxidation half. Textbook Solutions Expert Tutors Earn. Here it is in all its glory.
Fe2 MnO4- Fe3 Mn2 Acidic medium Al NaOH AlO2- H2 Basic medium Half reaction method. Balancing Redox Equations in Acidic Solution. A five step method for balancing redox equations called the half reaction method is used and after mastering these operations any chemical redox equation can be balanced by combining the five steps.
Solution for Balance the net ionic redox equations by the half-reaction method. For example C6H5C2H5 O2. Balancing a Net Ionic Redox Equation step-by-step 1.
This is balanced molecular equation. Assign oxidation numbers to all elements in the reaction. Balance the following equations of redox reactions.
Ad Browse Discover Thousands of Science Book Titles for Less. __Fe3 __NH3 __Fe2 __NO. Answer 1 of 2.
CrO42- C6H6 Cr3 _CO2 reductant. Balancing a redox equation in basic solution. Second the BrO ions react to form bromide ions and bromateV ions.
Full reaction balanced in acid. Draw a line connecting the atoms involved in oxidation and another line connecting the atoms involved in reduction. Molecular Ionic and Net Ionic Equations.
Here I have described the half reaction method. Balancing a Net Ionic Redox Equation step-by-step 1. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation breaking the equation into half reactions adding the electrons balancing the charges with the addition of hydrogen or hydroxide ions and then completing the equation.
You just have to insert the equation and the calculator will display oxidation and reduction. Balance the atoms in each half reaction. Write an unbalanced equation.
Separate the redox reaction into two half reactions. FREE Answer to balancing net ionic equations. Identify the species which undergoes oxidation and reduct.

Redox Reactions Part 4 Writing Net Ionic Equations Youtube

9 1 Writing Net Ionic Equations Sl Youtube
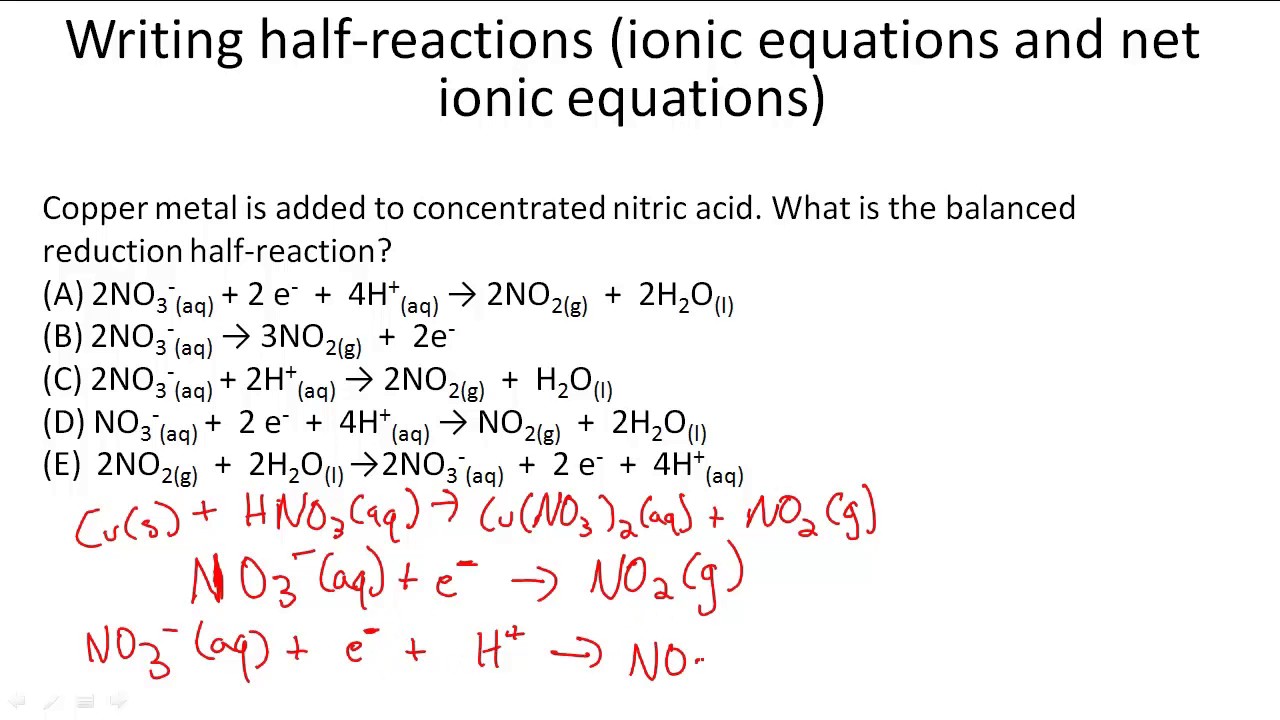
Writing Half Reactions Ionic Equations And Net Ionic Equations Youtube
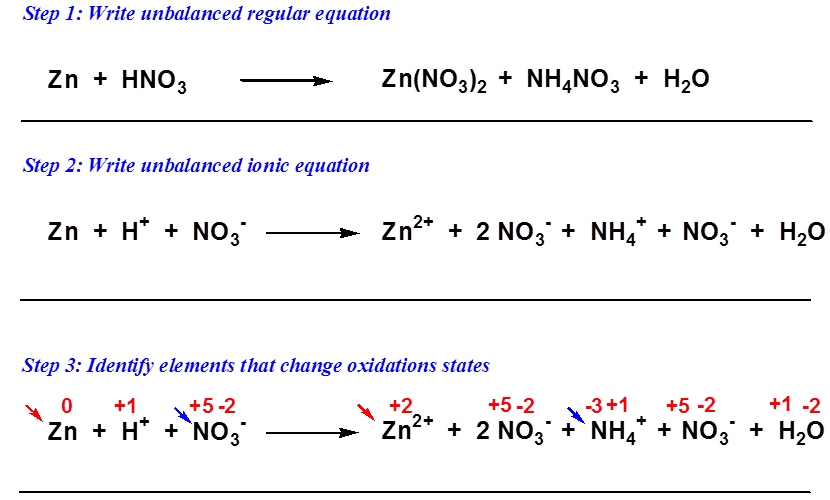
No comments for "Balancing Net Ionic Redox Equations"
Post a Comment